By Dr Marnie Graco, recipient of the 2022 Nancy Gray MND Postdoctoral Fellowship
Motor neurone disease (MND) causes the muscles to weaken, including the muscles involved in breathing. Weak respiratory muscles leads to symptoms such as fatigue, difficulty sleeping and shortness of breath. Research has shown that using overnight breathing support or “non-invasive ventilation” (NIV) improves quality of life and can extend life for people with MND. In fact, NIV is currently the most effective treatment for extending life in MND. Despite this, data from the Australian MND registry showed that from 2005 to 2015 just one in five people with MND in Australia tried NIV. Not everyone with MND will need or want to use NIV, but everybody should have the same opportunity to try it.
We recently conducted a survey which revealed 25% of respondents had never discussed NIV with a health professional. Furthermore, using NIV was related to where people live (including their state and remoteness), their gender, who they live with, and whether they attend an MND multidisciplinary clinic. This suggests there is inequitable access to NIV across Australia. We are delving deeper into this problem by interviewing people with MND, their families, and health professionals. The information we gather from these interviews will help us design solutions to help those with MND access NIV as part of their care.
What is NIV?
Non-invasive ventilation (NIV) is a machine that supports breathing by delivering pressurised air to the lungs via a face mask. It is sometimes called “Bi-PAP”, which stands for “bi-level positive airway pressure”. NIV is very different to “invasive ventilation”, which takes over breathing by inserting a breathing tube into the windpipe. NIV does not breathe for the person but rather helps our breathing muscles by pushing in a little extra air into the lungs as we breathe (similar to how an electric bike reduces the work of our leg muscles but still requires us to turn the pedals!)
NIV is also different from “CPAP” (continuous positive airway pressure) which is commonly used to treat sleep apnoea by splinting open the airway during sleep to prevent it from closing. While both NIV and CPAP deliver pressurized air via a facemask, CPAP delivers the same pressure of air during inspiration (breathing in) and expiration (breathing out). NIV, on the other hand, delivers a higher pressure of air during inspiration than expiration. This relieves the breathing muscles and improves breathing efficiency by helping the lungs take in more oxygen and remove carbon dioxide. NIV is usually offered to people with MND when their breathing muscles start to weaken, and they begin to experience symptoms such as breathlessness, fatigue, morning headaches and/or difficulty lying flat. It is mostly used overnight but can also be used during the day to relieve breathlessness and fatigue. As MND progresses, people often need to use NIV more and more throughout the day to help relieve their symptoms.
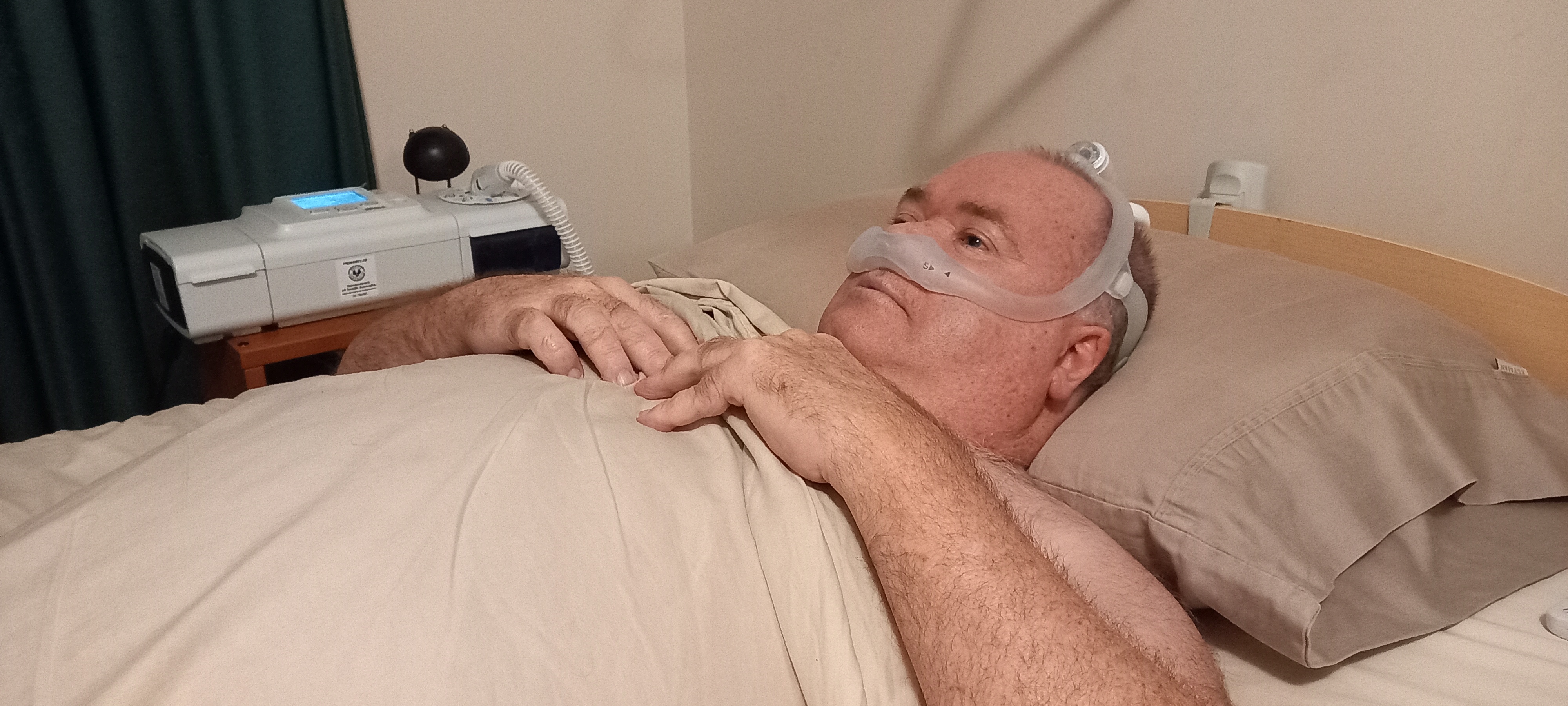 Greg getting set up with NIV
Greg getting set up with NIV
What are the benefits of NIV?
NIV is highly effective at relieving daytime symptoms such as breathlessness, fatigue, and morning headaches. MND research has shown that using NIV also slows the rate of respiratory decline, improves quality of life, and increases life-expectancy by up to 18 months. Some of these studies have also found that starting NIV early (i.e., when breathing symptoms first appear) and using NIV for at least 4 hours per night (on average) offers the greatest benefits to life expectancy. While not yet proven, some researchers also believe that using NIV may slow the progression of MND itself.
How well is NIV used?
Despite these well-known benefits of using NIV, data from the old Australian MND registry showed that between 2005 and 2015, only 20% of Australians with MND had tried NIV. In addition, research shows that of those who try NIV, less than 50% end up using it for the recommended 4 hours per night. Put together, these figures suggest that in 2015, less than 1 in 10 Australians with MND were adequately using NIV. These data are now about 10 years old, and other studies in the UK have found that NIV uptake and use has improved dramatically over the last 20 years. It is likely Australia’s has too. Estimates of NIV uptake and use in other countries are highly varied and difficult to compare due to the differences in how the data were collected. Recent studies from Ireland and the USA have reported that approximately 60-70% of their patients attending a MND multidisciplinary clinic used NIV, and of these, about 40-50% were using it for the recommended 4 hours per night. We will know more about Australia’s current uptake of NIV in a few years when the new MiNDAUS registry matures and acquires more data.
What are the barriers to using NIV?
For a person with MND, achieving the recommended “at least 4 hours of NIV per night” can be challenging. A combination of financial, social, cultural, and health system factors can affect the uptake and ongoing use of NIV. There are many hurdles and many reasons why some people with MND never achieve this. We have broken this complicated pathway into 3 main stages:
1. Accessing NIV services
Those with MND need to be offered NIV and referred to a service that can start them on NIV and support their ongoing use. Access to health care relies on the availability of suitable services as well as the ability to use those services. Not everyone with MND is able to access suitable NIV services, and not everyone is offered the opportunity to try. This can be highly dependent on where people live and the medical team managing their disease.
2. Accepting a trial of NIV
Accepting health care is the person’s informed choice to use it after carefully balancing the advantages and disadvantages. We are not suggesting that everyone with MND should accept a trial of NIV. The decision to try NIV is extremely personal, and deciding not to try NIV is a valid choice. However, we believe some people with MND are making this choice without being fully informed or for reasons that can be modified. For example, some people may choose not to try NIV because they lack enough support at home, or they haven’t been given accurate information about NIV. Providing more support and/or information could lower the barriers to accepting a trial of NIV.
3. Using NIV
Using NIV can be challenging, especially early on when the person/carer needs to learn how to use the equipment and get used to wearing a mask. People may experience problems such as air leaking from the mask, sore nose, dry eyes, blocked nose, or feelings of claustrophobia. It is normal to experience discomfort initially, but most problems can be overcome with a little trial and error and extra support from the health care team.
Our NIV research program
Our research group at the Institute for Breathing and Sleep in Melbourne are undertaking research to understand and address the common barriers at all three stages of the NIV journey (access, acceptance, and use).
We recently completed a survey of people living with MND in Australia about their NIV use. The survey was completed by 224 people with MND (or their carer), which represents about 10-15% of the total Australian MND population. Of those who responded, approximately 45% were using NIV and a further 10% been offered and accepted a trial of the therapy (that is, they had either started and stopped using NIV, or had been referred for NIV and were waiting to start). While we don’t know from this survey the true uptake of NIV across the country, the results suggest that NIV uptake has improved since last estimated in 2015.
Whilst promising, the survey results also indicate that access to NIV is not equal for all Australians. Our preliminary analysis found that:
- women and people living in rural and remote areas were less likely to be offered and accept NIV than men and those in regional or metropolitan areas;
- people living alone were less likely to use NIV than those living with family;
- people attending a specialised MND multidisciplinary clinic and under the age of 65 may be more likely to be using and offered NIV;
- people from Victoria were more likely to be using NIV than those in other states.
We are yet to fully understand these findings, and there are many limitations to our survey that need to be acknowledged. However, overall, these findings do support our “hunches” and we have theories that could explain these results. These hunches and theories are based on our clinical and lived experience of MND. For example:
- people living in rural areas may have to travel vast and prohibitive distances to access NIV;
- women may be more concerned than men about the additional “burden” of using NIV on their families;
- people living alone with limited hand function may not be able to put on and take off the mask on their own;
- people over 65 may have lower NIV uptake as a result of the NDIS rule that excludes people over 65 from accessing equipment and overnight support;
- people from Victoria may benefit from the well-coordinated and centralised MND clinic and state-wide NIV service.
These are just some of the theories that we are now exploring in our “qualitative research”. Qualitative research is a type of research that provides deeper insights into real-world problems. Instead of collecting numbers, qualitative research gathers participants' experiences and perceptions through conversations and/or observations. We are currently interviewing people with MND and their carers, their doctors and other health professionals to gain a much deeper understanding into why some people have trouble accessing and using NIV. This information, together with the results of the survey, will help us to design solutions that target the problems we find. Our aim is to make it easier for people with MND to access, accept and use NIV.
 Dr Marnie Graco discussing NIV use with Bruce and Natalie
Dr Marnie Graco discussing NIV use with Bruce and Natalie
Our research group are also leading a large research trial titled “A multi-centre randomised controlled trial of polysomnographic titration of NIV in MND” or “3TLA” trial. This trial is testing a new method of starting people with MND on NIV. As mentioned previously, NIV usage is often not used for the recommended 4 hours per night due to discomfort or intolerance. This trial involves monitoring NIV participants overnight in the hospital during a sleep study. Here the NIV settings are fine-tuned to optimise breathing support and make the NIV device more comfortable to use. Participants are randomly allocated to either a treatment or control group. The control group are still provided NIV and come in for a sleep study, but no changes are made to their NIV settings unless they express concerns/ask the clinician for changes to be made.
The 3TLA trial is being conducted across 7-sites in Australia at the hospitals listed below:
- Austin Hospital, VIC, Melbourne
- Macquarie University Hospital, NSW
- Westmead Hospital, NSW
- Royal Prince Albert Hospital, NSW
- The Prince Charles Hospital, QLD
- Sir Charles Garnier Hospital, Perth
- Flinders Medical Centre, Adelaide
How can you get involved in our research?
If you have MND or care for someone with MND and would like to participate in our interview study to share your experiences of accessing NIV, whether they be positive or negative, please reach out to our research team via email: nivinmnd.research@austin.org.au or phone/ text: 0468 862 693.
Similarly, if you would like to be part of the community to help design strategies to improve the uptake of NIV among Australians with MND, please contact us using the same details.
If you have been diagnosed with MND, have not yet started NIV, are living in one of trial site states listed above, and are interested in participating in the 3TLA trial, please speak to your neurologist about the 3TLA trial. More information can be found on the trial website: www.3TLA.org
To everyone who has already contributed to this research, by completing the survey or being interviewed or participating in the trial, thank you so much. We are 100% committed to using the information you generously gave us to make the NIV journey easier for people living with MND.
Where can you find out more about NIV?
Talk about NIV with your neurologist and other health professionals. There are also many great sources of information about breathing supporting, including NIV, written for people with MND. We have listed a few below:
https://www.mndaustralia.org.au/mnd-connect/living-with-mnd/breathing-and-mnd
https://mnddecisiontools.com/public/3/decision_tool
https://mybreathing.mymnd.org.uk/
https://www.mndassociation.org/support-and-information/living-with-mnd/breathing-and-ventilation




